1902 Encyclopedia > John Dalton
John Dalton
English chemist
(1766-1844)
JOHN DALTON (1766-1844), the celebrated physicist, and founder of the atomic theory of chemistry, was born September 5, 1766, at Eaglesfield, 2 3/4 miles south-west of Cockermouth, in Cumberland. His grandfather, Jonathan Dalton, was a member of the Society of Friends, and Dalton as well as his parents belonged to that body. His father, Joseph Dalton, who in 1755 married Deborah Greeup, had three children—Jonathan, John, the subject of this sketch, and Mary. The occupation in which he was engaged, namely, that of weaving woolens, was not a lucrative one, and Mrs Dalton assisted in the support of the family by the by the sale of stationery. John received his early education from his father and form a Mr Fletcher, the teacher of the Quaker’s school at Eaglesfield. At the age of twelve he himself began the work of school-teaching, in which he continued for two years; then, for a year or more, he worked occasionally on his father’s farm. His principal study was mathematics, in which he received aid from a distant relative, a gentleman of the name of Robinson, living in the vicinity of Eaglesfield. In 1781 Dalton left his native village to become assistant to his cousin George Bewley, the master of a school for boys and girls at Kendal; and there he spent the next twelve years of his life in teaching, and in studying Latin, Greek, mathematics, and natural philosophy. During that period he became acquainted with the blind philosopher, Mr. Gough, to whose influence and help his progress in scientific knowledge was in no small measure due. In 1785 Dalton became, through the retirement of his cousin, joint-manager with his brother of the school at Kendal, and in addition to his ordinary teaching he, in 1787 and 1791, gave courses of lectures in natural philosophy. The school was not generally popular, for its young masters were uncouth in manners, and kept aloof from society. Discipline was strict, and the elder brother Jonathan is said to have been stern and severe; John being milder and gentler, and continually preoccupied with mathematics, allowed faults to escape his notice, and was consequently preferred by the scholars. About the year 1790 Dalton appears to have been desirous to secure a larger sphere for his abilities by entering on the profession of law or of physic; but his projects meeting with no encouragement from his relations, he continued to live at Kendal, till in the spring of 1793 he obtained, mainly through Mr Gough, the appointment of teacher of mathematics and natural philosophy in the New College, Moseley Street, Manchester. That position he rerained up to the time of the removal of the Collage to York in 1799, when he became a private tutor. In 1794 the number of his pupils at the College, in mathematics, mechanics, algebra, geometry, book-keeping, natural philosophy, and chemistry, was 24. It was in 1792 that he first visited London, which he described as "a surprising place, and well worth one’s while to see once; but the most disagreeable place on earth for one of a contemplative turn to reside in constantly."

John Dalton
During his residence at Kendal Dalton had contributed solutions of problems and questions on various topics to the Gentleman’s and Ladies’ Diaries; but his first separate publication was his Meteorological Observations and Essays, published September 1793, a result of the study of natural phenomena during upwards of seven years previously. The book contained much original matter, but met nevertheless with only a limited sale, for, having been printed exclusively for the author, it never found its way in any large numbers into the hands of publishers. Another work by Dalton, entitled Elements of English Grammar, was published in 1801. On October 3, 1794, Dalton became a member of the Manchester Literary and Philosophical Society, before which, on the 31st, he read a communication entitled "Extraordinary facts relating to the Vision of Colours." In this paper he gives the earliest account of that ocular peculiarity known as dyschromatopsis, chromato-psendopsis (false vision of colours), Daltonism, parachromatism, or colour-blindness, and sums up its characteristics as observed in himself and others. [Footnote 784-1] When a boy, being present at a review of troops, and hearing those around him expatiating on the gorgeous effect of the military costume, he asked in what the colour of a soldier’s coat differed from that of the grass on which he trod; and it was the derisive laugh and the exclamations of his companions which this question called forth that first made him aware of the defectiveness of his sight. Besides the blue and purple of the spectrum he was able to recognize but one colour, yellow; or, as he states in his paper, "that part of the image which others call red appears to me little than a shade or defect of light; after that the orange, yellow, and green seem once colour, which descends pretty uniformity from an intense to a rare yellow, making what I should call different shades of yellow."
On March 1, 1799, Dalton read to the Manchester Society a paper on rain and drew, and the origin of springs, which was subsequently followed by various disquisitions—on heat, the colour of the sky, stream, the auxiliary verbs and participles of the English language, and the reflectibility and refrangibility of light. In May 1800 he was elected to the secretaryship of the society, an office which he held until 1808, when he became vice-president in the place of Dr Roget. In 1817 he became president, and remained so till the time of his death. On July 31, 1801, was read the first of four important essays by Dalton, "On the Constitution of Mixed Gases," "On the Force of Steam or vapour from Water and other Liquids in different Temperatures, both in a Torricellian Vacuum and in Air," "On Evaporation;" and "On the Expansion of Gases by Heat." In the second of these he makes the striking remark—"There can scarcely be a doubt entertained respecting the reducibility of all elastic fluids, of whatever kind, into liquids; and we ought not to despair of effecting it in low temperature, and by strong pressures exerted upon the unmixed gases;" further, he describes experiments to ascertain the tension of acqueous vapour at different points between 32º and 212º Fahr., and concludes, from observations on the behavior of the vapour of six different fluids, "that the variation of the force of vapour from all liquids is the same for the same variation of temperature, reckoning from vapour of any given force." In the fourth essay he observes—"I see no sufficient reason why we may not conclude that all elastic fluids under the same pressure, expand equally by heat, and that for any given expansion of mercury, the corresponding expansion of air is proportionally something less, the higher the temperature….. It seems, therefore, that general laws respecting the absolute quantity and the nature of heat are more likely to be deprived from elastic fluids than from other substances." Dalton thus both enunciated the law of the expansion of gases, stated six months later by Gay-Lussac, and indicated the future employment of the air-thermometer.
But the most important of Dalton’s numerous investigations are those concerned with the atomic theory of chemistry. The subject of chemistry seems to have first occupied his attention his attention about the year 1796. In 1802 he had already arrived at some conception of the law of the multiple combining proportions of the elements, which was afterwards developed by him. Thus, in a paper "On the Proportion of the Several Gases or Elasatic Fluids constituting the Atmosphere," read on the 29th of October in that year, he says—though, as it happened, his conclusions were based upon the incorrect supposition that the size of the vessels he employed affected the nature of the chemical union of the gases they contained—"the elements of oxygen may combine with a certain portion of nitrous gas, or with twice that portion, but with no intermediate quantity. In the former cases nitric acid is the result, in the latter nitrous acid."
Dr Thomson states (History of Chemistry, vol. ii.)—"Mr Dalton informed me that the atomic theory first occurred to him during his investigations of olefiant gas and carburetted hydrogen gas." In 1850, however, in a notice of Wollaston, read before the Glasgow Philosophical Society, he remarks,—"Mr Dalton founded his theory oil the analysis of two gases, namely, protoxide and deutoxide of azote . . . . . The first of these he considered as a compound of one atom of azote with one atom of oxygen, and the second of one atom of azote united with two atoms of oxygen." Inasmuch as from the recognition of the law of definite and multiple combining proportions of the elements originated the establishment of that of their relative, Dalton may be said to have received assistance in the foundation of his atomic theory from the researches here alluded to by Thomson; but the latter part of the statement is manifestly erroneous, for the two gases referred to were invariably represented by Dalton as compounds respectively of two atoms and one atom of azote (nitrogen) with a single atom of oxygen. It is doubtless the earlier of Thomson’s observations that is to be regarded as correct, more especially as Dalton himself says, in 1810, in his New System of Chemical Philosophy, with respect to carburetted hydrogen,—"No correct notion of the constitution of the gas about to be described seems to have been formed till the atomic theory was introduced and applied in the investigation. It was in the summer of 1804 that I collected, at various times and in various places, the inflammable gas obtained from ponds." As a matter of fact, the first germs of the atomic theory were Dalton’s views of the separate existence of aqueous vapour in the atmosphere, which necessitated the assumption that gases were constituted of independent atoms; indeed they are represented as such, each atom having its distinguishing symbol, in the plate accompanying the paper "On the Constitution of Mixed Gases." Dalton appears already in 1803 to have pictured to himself the form of atoms, for in a paper "On the Absorption of Gases by Water" we read—" A particle of gas pressing on the surface of water is analogous to a single shot pressing on a square pile of them;" and five years later, he writes in his New System,—"Whatever, therefore, may be the shape or figure of the solid atom abstractedly, when surrounded by such an atmosphere [of heat] it must be globular; but as all the globules in any small given volume are subject to the same pressure, they must be equal in bulk, and will, therefore, be arranged in horizontal strata like a pile of shot." At the end of the paper on "Absorption" just alluded to, Dalton gives the following first table of the relative weights of the ultimate particles of gaseous and other bodies, which was constructed, he tells us, in order to test whether the solubility of gases in water was dependent upon the weight of their particles:—

[Footnote 785-1 (referring to Nitrous oxide in the table above)]
As this table contains the results of the analyses of olefiant gas and carburetted hydrogen made in the summer of 1804, it must have been completed after that date, and possibly was not added to the paper containing it till shortly before the printing of the latter in November 1805. It was in 1803, as we are informed in the preface to the New System, that Dalton "was gradually led to those primary laws which seem to obtain in regard to heat and to chemical combination; " and in a letter to his brother in that year be writes that he has been fully engaged in all his leisure hours in the pursuit of chemical and philosophical inquiries, "having got into a track that has not been much trod in before." Dr Bryan Higgins, in a little pamphlet composed about the year 1775, had treated of "atoms" which united with one another; but the fixity of the constitution of chemical substances had apparently formed no part of his ideas. "The matter of fire," according to him, "limits the quantity in which aeriform fluids, and bodies containing it, can combine chemically," and it is his belief that the forces of atoms measure the attraction of matter, yet he ventures on no deduction as to the comparative numbers of the attracting atoms. Upon these views we find an advance in the writings of William Higgins, who not only held that atoms combined to form molecules of compound bodies, but reasoned that they must unite singly or by twos and threes, there being no intermediate division of atoms; nowhere, however, does he attempt to elevate his conclusions into a general law. Next Richter, and after him Fischer, showed the existence of definite quantitative relations between the constituents of bodies, but for these relations they assigned no cause; and it was reserved for Dalton to give to the world a theory which linked together and reduced to order and simplicity the previously disconnected and unexplained phenomena of chemical combination. Till 1811 Dalton, who drew his deductions from his own rough experimental work, was unacquainted with the observations of William Higgins; and although Richter’s determinations may have aided him in the proving of his laws, still, as Dr R. A. Smith has remarked, "they could never have given him fundamental ideas." Dalton makes the following clear distinction between his own researches with respect to the ultimate constitution of matter and those of other chemists (New System, pt. i. p. 213, 1808):—
"In all chemical investigations, it has justly been considered an important object to ascertain the relative weights of the simples which constitute a compound. But unfortunately the inquiry has terminated here; whereas from the relative weights in the mass, the relative weights of the ultimate particles or atoms of the bodies might have been inferred, from which their number and weight in various other compounds would appear, in order to assist and to guide future investigations, and to correct their results, Now, it is one great object of this work to show the importance and advantage of ascertaining the relative weights of the ultimate particles, both of simple and compound bodies, the number of simple elementary particles which constitute one compound particle, and the number of less compound particles which enter into the formation of One more compound particle.
If there are two bodies, A and U, which are disposed to combine, the following is the order in which the combinations may take place, beginning with the most simple, namely:—
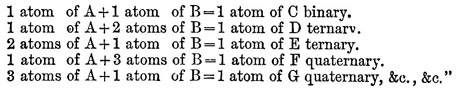
In 1810 appeared the second part of volume i. of the New System, in which the chemical elements are described. The first part of volume ii. was not published till 1827 ; it by no means represents the advanced state of chemical science at that time, and the appendix, giving Dalton’s latest views, is the only portion of it that is of any special interest. A history of the development of the atomic theory since its first promulgation will be found under CHEMISTRY, vol. V. p. 465. By Dr Thomson, its first advocate, by Wollaston, and by Dr Henry, it was ably supported, and the analyses of Berzelius placed it on a stable footing. "The theory of multiple proportions," wrote Berzelius, "is a mystery without the atomic hypothesis." Strange to say, the conclusions of Gay-Lussac with regard to the combining volumes of gases, which afforded the strongest evidence in favour of the atomic theory, were distrusted, and perhaps never fully accepted by Dalton. The tenacity with which he clung to opinions once formed is further exemplified by his unwillingness to recognize chlorine as a chemical element, and his persistent use of the atomic weights first adopted by him, in spite of the later and more trustworthy determinations of other chemists. The memoirs of Dalton read before the Manchester Literary and Philosophical Society were in all 116. In one of these, read in 1814, he lays down the principles of the volumetric method of analysis, of which he is undoubtedly to be regarded as the originator, although its wide practical application is the result of the labours of numerous after-chemists. The earlier of Dalton’s papers are the most important and complete; one of his latest, however, "On a New and Easy Method of Analyzing Sugar," describes a discovery of much interest, viz., that the volumes of highly hydrated salts when dissolved are equal to those of their volumes of water, the volume of the salt itself disappearing. Before Dalton had become known as the propounder of the atomic theory, he had already attained a considerable reputation by his scientific labours, and in 1804 he was chosen to give a course of lectures on natural philosophy at the Royal Institution in London. Subsequent discourses were delivered by him at the same place in the winter of 1809-10. He was, it would seem, deficient in many of those qualities that go to form an attractive public lecturer. His voice is said to have been harsh, indistinct, and unemphatic, and his manner of dealing with his subject ineffective; be is described, moreover, as an indifferent experimenter, and as "singularly wanting in the language and power of illustration." An imaginative or brilliant style of diction, it is to be supposed, can scarcely have been at the, command of one whose hours of leisure from the routine of tuition were unceasingly devoted to laboratory work, and who eschewed, and even to some extent discouraged, literary pursuits. His library, he was once heard to declare, be could carry on his back, and yet he had not read half the books which constituted it. In the autumn of 1805 Dalton went to live in George Street, Manchester, with his friend the Rev. W. Johns, and with him and his family he continued to reside, in the greatest harmony, for the next twenty-six years. Engaged in his favourite studies, be passed a quiet and almost uneventful life, interrupted only by occasional visits to London and other cities, and by annual excursions to the Lake country. Into society he rarely went, and amusement he had none, with the exception of a game at bowls on Thursday afternoons. In 1810 he was asked by Davy to offer himself as a candidate for the fellowship of the Royal Society, but he declined, possibly from pecuniary considerations. In 1822 he was proposed without his knowledge, was elected, and paid the usual fee. Four years later he received the king's medal of the society, "for the development of the chemical theory of Definite Proportions usually called the Atomic Theory, and for his labours and discoveries in physical and chemical science."
In the summer of 1822, in company with Mr Benjamin Dockray and Mr W. D. Crewdson, Dalton spent a short time at Paris, where he met Ampère, Arago, Berthollet, Biot, Brèquet, Cuvier, Fourier, Gay-Lussac, Laplace, Thénard, Vauquelin, and other distinguished men of science. Six years previously he had been made a corresponding member of the Academy of Sciences; and in 1830 he was elected by that body to fill the place of Davy as one of its eight foreign associates. Dalton was present at the first meeting of the British Association, held at York in 1831. On the occasion of the second meeting, at Oxford in 1832, the honorary degree of D.C.L. was conferred upon him. The scarlet hue of his doctor's gown was to him, he said, "that of nature," the colour of "green leaves."
In June 1833, Lord Grey’s Government conferred upon Dalton an annual pension of £150, which in 1836 was raised to £300. In the former year a subscription list was opened in Manchester to obtain funds for providing that city with a lasting memorial of its great chemist; and the sum of £2000 having been raised, Chantrey was intrusted with the execution of a bust, which was eventually placed in the entrance hall of the Manchester Royal Institution. During his stay in London, whither he had gone in 1834 to sit to the sculptor, Dalton was presented at court, and in the autumn he received from the university of Edinburgh the degree of LL.D. He officiated as vice-president of the chemical section of the British Association at Dublin in 1835, and at Bristol in 1836. On the 18th April 1837, he was seized with an attack of paralysis, a disease of which his brother had died in December 1834. In the following year, on the 15th February, he had a second attack, after which, though still able to make experiments, he was manifestly much enfeebled, both physically and mentally, and required constant medical attendance. On May 20, 1844, he suffered from another fit. On the 26thof July 1844, he recorded, with trembling hand, his last meteorological observation, and on the morning of the 27th he fell from his bed, and was found lifeless by his attendant. He was publicly buried on the 12th of August at Ardwick cemetery, about a mile and a half from Manchester.
In person Dalton was robust and muscular, and his countenance was open, and expressive of the earnestness, simplicity, and truth of his character. His height was about 5 feet 7 inches; he stooped slightly; and his gait was stiff and awkward, but rapid. In dress he adhered to the mode of the Quakers. His manners were singularly free from pedantry and ostentation, and he had a grave, quiet demeanour. Generally he enjoyed excellent health. His medical attendant, finding him once greatly recovered from an attack of catarrh, attributed the improvement to a dose of James’s powder prescribed on the previous day. "I do not well see how that can be," said Dalton, "as I kept the powder until I could have an opportunity of analyzing it," Dalton was somewhat silent in general company, but with his familiar friends he would often indulge in active conversation. His letters to his acquaintances evince no small power of observation. On religious topics he appears to have been peculiarly reserved, and his friends found it difficult to gain an idea of his doctrinal views. He "never had time" to get married, he said; but his correspondence, and the testimony of those who knew him, show that he delighted in the society of women of education and refinement; his pinched circumstances in early life were perhaps the chief cause why he remained single. He liked tobacco, and remarked of Davy, "the principal failing in his character as a philosopher is that lie does not smoke." Dalton was careful, though not parsimonious, in his expenditure, and left at his death a small fortune; when occasion required he could show himself remarkably open-handed. Davy wrote of Dalton in 1829:—"He was a very coarse experimenter, and almost always found the results he required, trusting to his head rather than to his hands. Memory and observation were subordinate qualities in his mind; he followed with ardour analogies and inductions; and however his claims to originality may admit of question, I have no doubt that he was one of the most original philosophers of his time, and one of the most ingenious." Superadded to his natural talents, and "his almost intuitive skill in tracing the relations of material phenomena," there was in Dalton, to use the words of Professor Sedgwick, "a beautiful moral simplicity and singleness of heart, which made him go on steadily in the way he saw before him, without turning to the right hand or to the left, and taught him to do homage to no authority before that of truth."
Henry, Life of Dalton, Cavendish Society, 1854; Robert Angus Smith, Memoir of John Dalton and History of the Atomic Theory, 1856. A list of Dalton's papers and other publications is given on pp. 253-63 of the latter work. See also Roscoe, "On Dalton’s First Table of Atomic Weights," in Nature, Nov. 19, 1874. (F. H. B.)
Footnotes
784-1 The subject is fully treated of in Dr G. Wilson’s Researches on Colour-Blindness, 1855.
785-1 The figures for nitrous gas (nitric oxide) and nitrous oxide should have been 9·7 and 13·9, i.e., 5·5+4·2 and 5·5+4·2x2.
The above article was written by F.H. Butler, Associate of the Royal School of Mines.
|